 Internal Quality Control Programs for Neonatal Screening Tests
Internal Quality Control Programs for Neonatal Screening Tests
The internal quality control (IQC) is very important for the laboratory quality assurance system. To assist newborn screening laboratories in maintaining the high quality of screening tests, Preventive Medicine Foundation provides “Internal Quality Control Programs for Neonatal Screening Test” for laboratories to monitor with-in laboratory test quality performance.
Independent third-party materials are used in these IQC programs which could assist newborn screening laboratories meet the requirements recommended by international standards (cf. ISO 15189 and CLSI C24-A3: 2006) that the IQC materials need to be different from the calibrator materials and reagent kits to ensure that the QC procedure provides an independent assessment of system performance.
-
Homogeneity and Stability conform to ISO 13528 Annex B.

-
Two concentration or activity levels of filter papers (one filter paper each) .
-
The quality control materials are prepared from human whole blood . Control ranges compatible with range in neonatal bloods..
-
Online MIS systems provide the real-time and long-term cumulative statistic result.
-
Participants can also use these results for inter-laboratory comparison with other participating laboratories.
-
The certificate of participation ( or annual certification label ) will be issued to whom has participated in the IQC program of the year and reported results more than 9 months.

![]() IQC program for Neonatal G6PD Screening
Test
IQC program for Neonatal G6PD Screening
Test
![]() IQC program for Neonatal Screening
Tests by Tandem Mass Spectrometry
IQC program for Neonatal Screening
Tests by Tandem Mass Spectrometry
![]() IQC
program for Neonatal TSH Screening Test
IQC
program for Neonatal TSH Screening Test
![]() IQC
program for Neonatal Phe Screening Test
IQC
program for Neonatal Phe Screening Test
![]() IQC
program for Neonatal 17-OHP Screening Test
IQC
program for Neonatal 17-OHP Screening Test
For more details, please contact us < nsiqc@pmf.tw >
 Internal Quality Control Programs for G6PD
Quantitative Tests
Internal Quality Control Programs for G6PD
Quantitative Tests
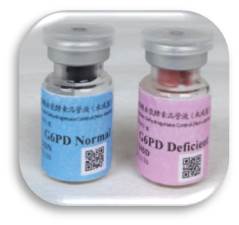
This IQC program provides two levels of G6PD quality control materials to the clinical laboratories to be used in routine IQC for G6PD quantitative test. The lyophilized QC materials were prepared from human red blood cells G6PD. The blood used to prepare the QC materials was tested free from HBsAg, Anti-HCV, Rapid Plasma Reagin (RPR or TPPA), Anti-HIV and Anti-HTLV. This IQC system uses third-party QC materials independent from the reagent kits ( according to ISO 15189 and CLSI C24-A3: 2006) .
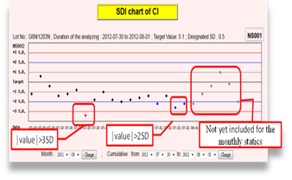
As soon as participants submit the IQC results online, the real-time statistic results (including mean, SD, CV, TE and σ) and Shewhart QC Chart will be available immediately.
-
Homogeneity and Stability conform to ISO 13528 Annex B.

-
Two activity levels.
-
The quality control materials are prepared from human whole blood, no extra G6PD added.
-
Online MIS systems provide the real-time and long term cumulative statistic result.
-
Participants can also use these results for inter-laboratory comparison with other participating laboratories.
-
The certificate of participation ( or annual certification label ) will be issued to whom has participated in the IQC program of the year and reported results more than 9 months.
![]() IQC program for G6PD Quantitative
Test
IQC program for G6PD Quantitative
Test
For more details, please contact us < g6pd@g6pd.tw >